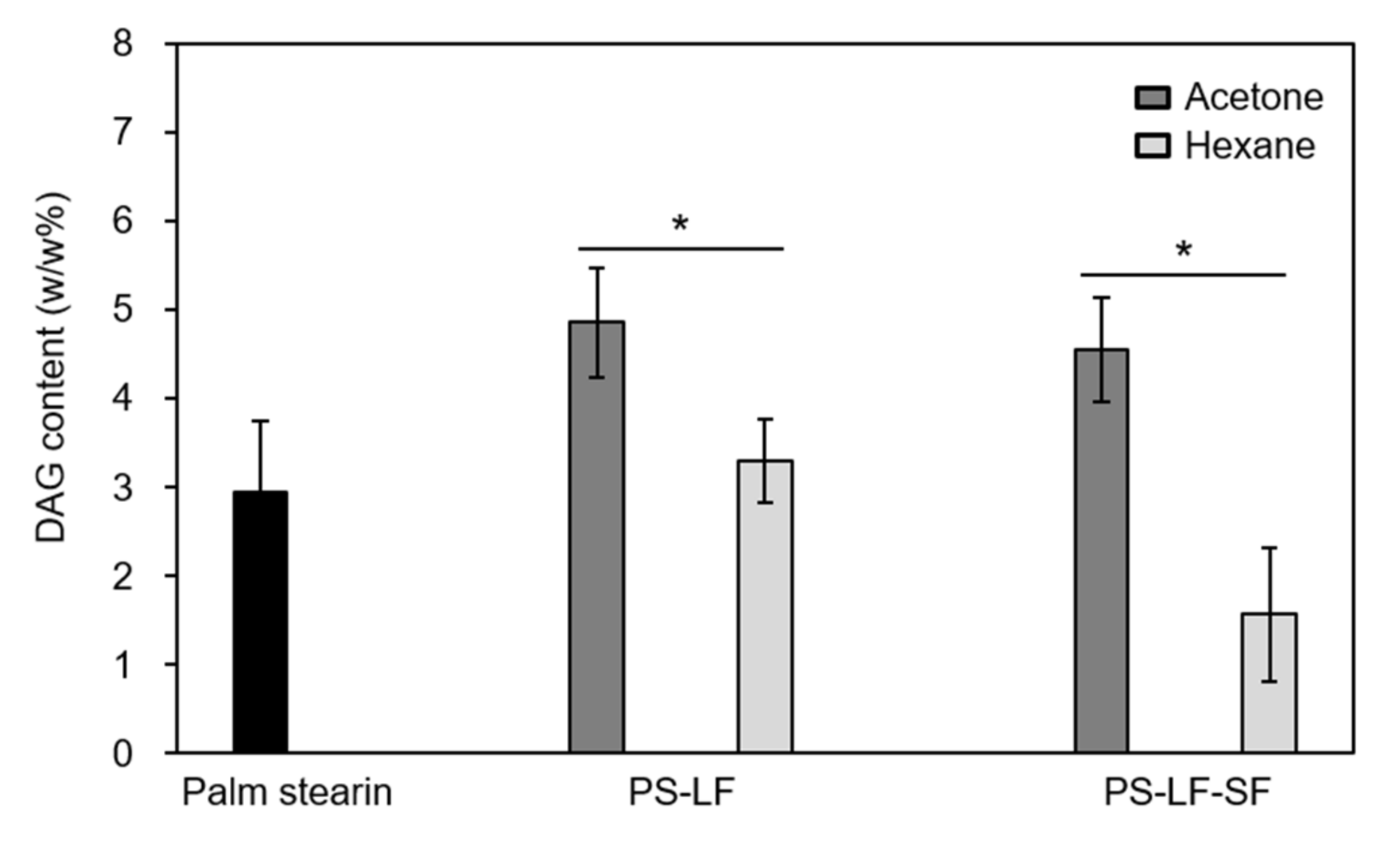
Molecules | Free Full-Text | Preparation of Low-Diacylglycerol Cocoa Butter Equivalents by Hexane Fractionation of Palm Stearin and Shea Butter

Separation of azeotropic mixture acetone + hexane by using polydimethylsiloxane membrane - ScienceDirect

Separation of azeotropic mixture acetone + hexane by using polydimethylsiloxane membrane - ScienceDirect
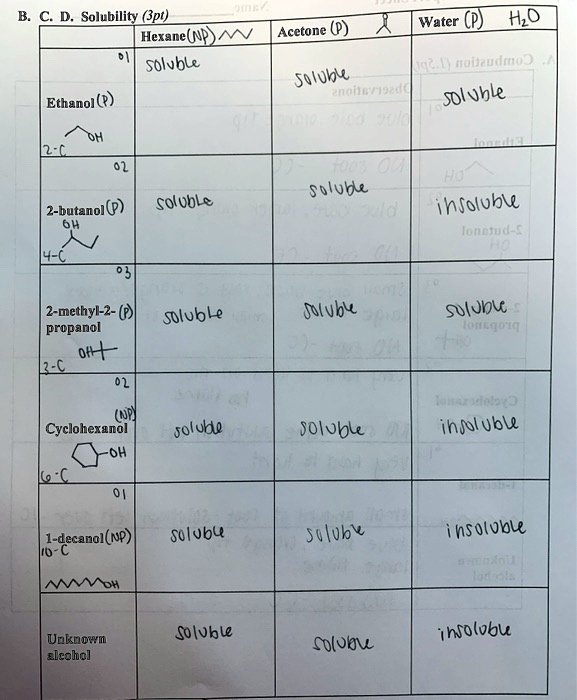
SOLVED: Text: Solubility (pt) Hexane (Not Soluble) Water (Soluble) Acetone ( Soluble) Ethanol (Soluble) 2-butanol (Soluble) 2-methyl-2-propanol (Soluble) Cyclohexanol (Soluble) 1-decanol (Not Soluble) Unknown alcohol (Soluble)
Solubilities of some normal saturated and unsaturated long-chain fatty acid methyl esters in acetone, n-hexane, toluene, and 1,2-dichloroethane | Journal of Chemical & Engineering Data

Draw the structures of ethanol, acetone, toluene, hexane, and water. Classify each solvent as polar, nonpolar, or moderately polar. | Homework.Study.com

Effect of solvent polarity and adsorbed water on reaction between hexyltriethoxysilane and fumed silica - ScienceDirect
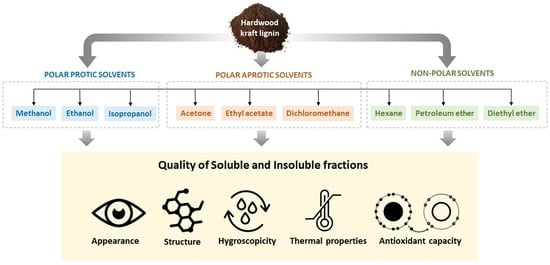
Polymers | Free Full-Text | One-Step Lignin Refining Process: The Influence of the Solvent Nature on the Properties and Quality of Fractions

ONE Wu De Ils concentration? 23 Suggest the important of intermolecular attractive interaction the following pairs. (1) n-hexane and n-octane (11) I, and CCI, (iii) NaClo, and water (iv) methanol and acetone (

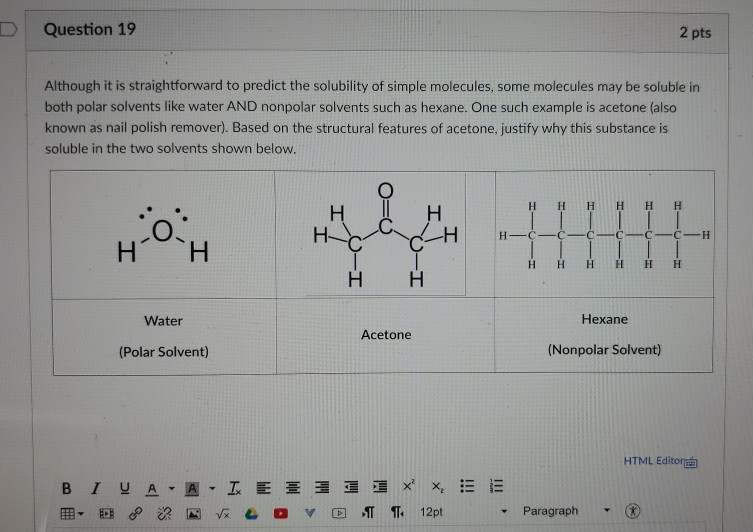
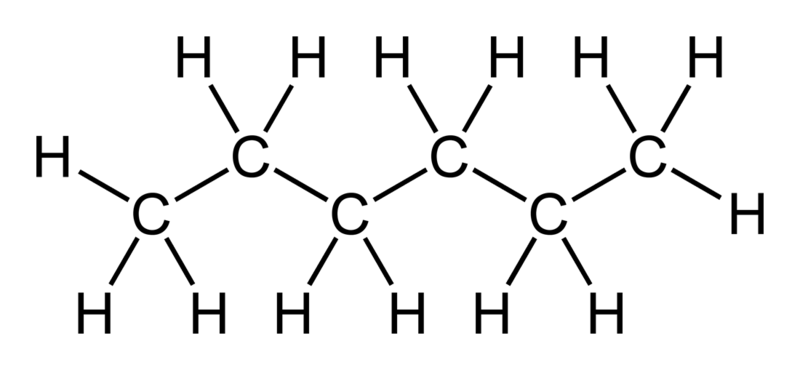
SOLVED: Although it is straightforward to predict the solubility of simple molecules, some molecules may be soluble in both polar solvents like water and nonpolar solvents such as hexane. One such example
![SOLVED: Acetone [(CH3)C=O] is a useful solvent because it dissolves a variety of compounds well. For example, both hexane [CH3(CH2)4CH3] and H2O are soluble in acetone. Explain why these solubility properties are SOLVED: Acetone [(CH3)C=O] is a useful solvent because it dissolves a variety of compounds well. For example, both hexane [CH3(CH2)4CH3] and H2O are soluble in acetone. Explain why these solubility properties are](https://cdn.numerade.com/ask_images/9d349811a4444b3a91bc31884ae75de9.jpg)
SOLVED: Acetone [(CH3)C=O] is a useful solvent because it dissolves a variety of compounds well. For example, both hexane [CH3(CH2)4CH3] and H2O are soluble in acetone. Explain why these solubility properties are